silver abbreviated electron configuration|ground state electron config : Tagatay Atomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable . Tingnan ang higit pa #m4maths Asked in "CTS" exam. Q. Find the odd one a) adg b) hkn c) psw d) mps for check solution visit given link -.
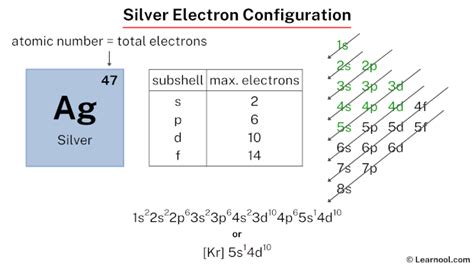
silver abbreviated electron configuration,The ground-state electron configuration of silver is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s1. This electron configuration shows that the last shell of silver has an electron and the d-orbital has a total of ten electrons. Therefore, the valence electronsof silver are one. The elements that form bonds by donating . Tingnan ang higit paground state electron configThe total number of electrons in silver is forty-seven. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in silver in specific rules in different orbits and orbitals is called the electron configurationof . Tingnan ang higit pasilver abbreviated electron configuration ground state electron configThe total number of electrons in silver is forty-seven. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in silver in specific rules in different orbits and orbitals is called the electron configurationof . Tingnan ang higit paAtomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable . Tingnan ang higit pa
Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electronsof the atom revolve around the nucleus in a certain circular path. These circular . Tingnan ang higit pa Mar 23, 2023 To write the configuration for the Silver and the Silver ion, first we need to write the electron configuration for just Silver (Ag). We first need to find the number of electrons for the. The silver electron configuration, represented as [ Kr] 5s 1 4d 10 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 1 4d 10, illustrates the precise arrangement of electrons within the atom.

The electron configuration of silver is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s1. Silver has various uses, mainly in commercial, industrial and personal procedures. Silver’s .
In this article, we will describe the various facts of Ag electronic configuration like Ag electronic configuration notation, unabbreviated electronic . Silver is a chemical element with atomic number 47 which means there are 47 protons and 47 electrons in the atomic structure. The chemical symbol for Silver is .Electron configuration of Silver. Abbreviated form: [Kr] 4d10 5s1 . Ionization Energies of Silver. The following table lists the ionization energies IE (ionization potentials); the IE is .

The electron configuration for silver (Ag) is based upon the place meant of silver in the fifth row of the periodic table in the 11th column of the periodic table or the 9th column of the . The electronic configuration of periodic elements shows the total number of electrons arranged in their atomic orbital. Let us see the electronic configuration of Ag. Electronic configuration of Ag is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 1. Silver is the transition metal atom its symbol is Ag. It is the 47 th periodic table element, .The electron configuration of silver is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s1. Silver has various uses, mainly in commercial, industrial and personal procedures. Silver’s resistance to corrosion allows it to be useful in creating special containers or in protecting other metals. Silver is characterized by being a metal with a grayish and .silver abbreviated electron configuration Add an electron to the anion electron configuration. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. For Cl −, it will be 1s²2s²2p⁶3s²3p⁶. Remove the outermost electrons in the cation, e.g. electron configuration for Mg 2+ will be 1s²2s²2p⁶.
Inner transition elements are metallic elements in which the last electron added occupies an f orbital. They are shown in green in Figure 3.4.6 3.4. 6. The valence shells of the inner transition elements consist of the ( n – 2) f, the ( n – 1) d, and the ns subshells. There are two inner transition series: But, the orbitals overlap. The Madelung rule gives the order: 1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p < 6s < 4f < 5d < 6p < 7s < 5f < 6d < 7p. Oganesson (element 118) is a good example to show the order of the orbitals. Its electron configuration is: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d .Introduction to electron configurations. Electron configurations describe where electrons are located around the nucleus of an atom. For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s subshell. Created by Sal Khan.For hydrogen, therefore, the single electron is placed in the 1s orbital, and the electron configuration . Silver: Z:47 [Kr] 5s 1 4d 10: Period 6: Period 7: Lanthanum: Z:57 [Xe] 6s 2 5d 1: . thus it can be used as a "shorthand" or abbreviated method for writing all of the electron configurations after 1s. 4. Identify the following elements: To write condensed electron configurations (also called abbreviated electron configurations) for elements we first write the full electron configuration for . The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number .The electron configuration for silver (Ag) is based upon the place meant of silver in the fifth row of the periodic table in the 11th column of the periodic table or the 9th column of the transition metal or d block. Therefore the electron configuration for silver must end as $$ 4s^9 $$ $$ 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^{10} 4p^6 5s^2 4d^9 $$ T his notation .
The abbreviated electronic configuration of Silver is [Kr] 4d10 5s1. To form abbreviated notation of electronic configuration, the completely filled subshells are replaced by the noble gas of the preceding period in .
Follow these steps to write abbreviated electron configurations. Step 1 Find the symbol for the element on a periodic table.. For example, to write an abbreviated electron configuration for zinc atoms, we first find Zn on the periodic table (see below).Step 2 Write the symbol in brackets for the noble gas located at the far right of the preceding . To write the configuration for the Silver and the Silver ion, first we need to write the electron configuration for just Silver (Ag). We first need to find . Helium atoms have 2 electrons. Both electrons fit into the 1s subshell because s subshells can hold up to 2 electrons; therefore, the electron configuration for helium atoms is 1s 2 (spoken as “one-ess-two”). Different subshells hold a different maximum number of electrons. Any s subshell can hold up to 2 electrons; p, 6; d, 10; .The full electron configuration of Mercury is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10. Mercury’s atomic number is 80, which means it has 80 electrons. To make the simplified or abbreviated electron configuration, the most essential part is to distribute the electrons which are in the valence shell, which means you are .
Inner transition elements are metallic elements in which the last electron added occupies an f orbital. They are shown in green in Figure 5.1.6 5.1. 6. The valence shells of the inner transition elements consist of the ( n – 2) f, the ( n – 1) d, and the ns subshells. There are two inner transition series: Referring to either Figure 6.4.3 6.4. 3 or 6.4.4 6.4. 4, we would expect to find the electron in the 1 s orbital. By convention, the ms = +1 2 m s = + 1 2 value is usually filled first. The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2.
silver abbreviated electron configuration|ground state electron config
PH0 · unabbreviated electron configuration for barium
PH1 · silver condensed electron configuration
PH2 · noble gas abbreviated electron configuration
PH3 · how to find electron configuration of element
PH4 · ground state electron config
PH5 · electron configuration finder
PH6 · abbreviated electron configuration chart
PH7 · abbreviated electron configuration cesium
PH8 · Iba pa